

The H−O−H bond angle is approximately 113°, and the center of mass is very close to the oxygen atom.
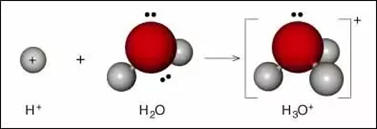
As shown in the images above, H 3O + has a trigonal pyramidal molecular geometry with the oxygen atom at its apex. Since O + and N have the same number of electrons, H 3O + is isoelectronic with ammonia. For example, a protonated hydroxyl group is an oxonium ion, but not a hydronium ion. Hydroxonium may also be used unambiguously to identify it.Īn oxonium ion is any ion with a trivalent oxygen cation. Nomenclature Īccording to IUPAC nomenclature of organic chemistry, the hydronium ion should be referred to as oxonium. A pH value less than 7 indicates an acidic solution, and a pH value more than 7 indicates a basic solution. At 25 ☌ (77 ☏), pure water has a pH of 7 and a pOH of 7 (this varies when the temperature changes: see self-ionization of water). In pure water, there is an equal number of hydroxide and H + ions, so it is a neutral solution. The molecules in pure water auto-dissociate into aqueous protons and hydroxide ions in the following equilibrium: The concentration of hydroxide ions analogously determines a solution's pOH. The concentration of hydronium or H + ions determines a solution's pH according to For this reason, it has been suggested that wherever possible, the symbol H +(aq) should be used instead of the hydronium ion. Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form. Three main structures for the aqueous proton have garnered experimental support: The Eigen cation, which is a tetrahydrate, H 3O +(H 2O) 3 the Zundel cation, which is a symmetric dihydrate, H +(H 2O) 2 and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H +(H 2O) 2(H 2O) 4. In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous H + and conjugate base. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton (a positive hydrogen ion, H +) to the surrounding water molecules ( H 2O). In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation H 3O +, the type of oxonium ion produced by protonation of water.


 0 kommentar(er)
0 kommentar(er)
